The third phase of the clinical trials of the Indian vaccine Covaxin has come to an end, the results of which proved that it protected about 77.8% of patients from an explicit form of COVID-19 infection, and about 65% from the delta variant of SARS-CoV-2, Report informs, citing TASS.
As researchers maintain, patients tolerated the vaccine well, and no major safety concerns were registered.
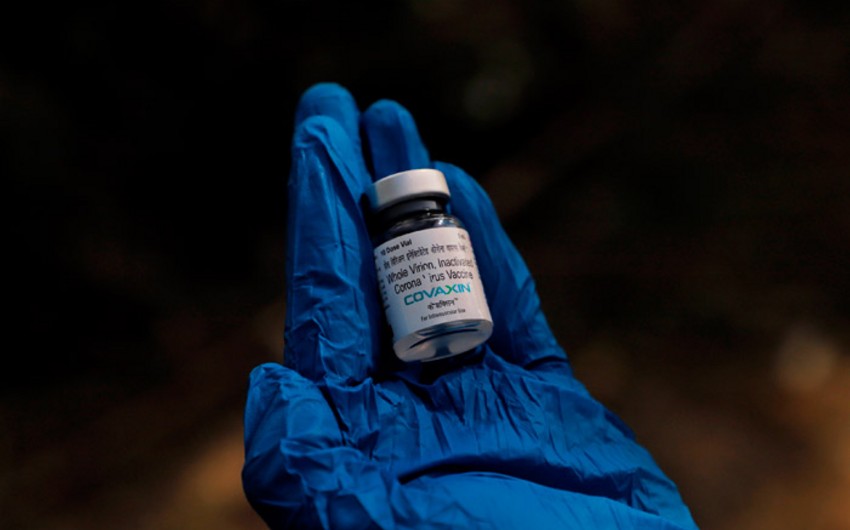
Covaxin (BBV152) is being developed by Bharat Biotech. This is a classic vaccine based on a weakened version of the SARS-CoV-2 virus, which poses no threat to the human body, but at the same time, helps the immune system to "remember" the real pathogen of COVID-19. Clinical trials of the drug began in July 2020.
Nearly 26,000 people, who received two doses of the vaccine every month, were involved in the recent clinical trials.


 https://static.report.az/photo/356e6441-66cb-3581-aa89-d0edc95a8e87.jpg
https://static.report.az/photo/356e6441-66cb-3581-aa89-d0edc95a8e87.jpg

